
Abdominal anatomy—the foundation of clinical practice
Introduction
Conventional descriptions of abdominal anatomy depict a complex system (1). They do not match modern day abdominal surgery nor can they explain the radiological appearance of the abdomen (2-4). Recent advances in abdominal anatomy demonstrate it is far simpler than previously thought (5,6). These advances are summarised in a description called the Mesenteric Model (MM) of abdominal anatomy (5). This model matches clinical observations and can be taken forward into all branches of clinical practice.
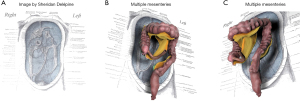
The conventional (peritoneal-based) model of abdominal anatomy forced the reader to think in terms of multiple, discrete mesenteries. These were termed the small intestinal mesentery, the transverse mesocolon and the sigmoid mesentery (Figure 1). Also, each of these mesenteries was described as inserting or attaching to the posterior abdominal wall along a “root”. That model predicts beginning and end points for each mesentery (i.e., anatomical limits). These are not clinically apparent. The conventional model was complex and lacked an explanation as to how it developed from the earliest to final form. Yet, it remained the model on which all teaching of abdominal anatomy was based for over 150 years and is still taught by anatomists. One of the main reasons for this was that an alternative model was never articulated. This meant there was no alternative with which to substitute the peritoneal model. Recently, the MM was described (5). This matches surgical and radiological observations and is fully explained by a developmental program (Figure 2).
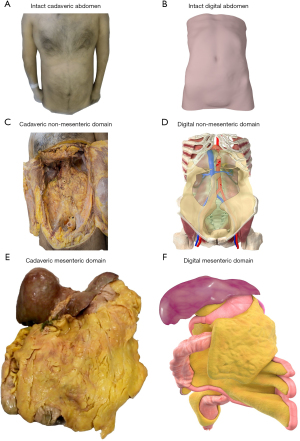
In the MM, the abdomen comprises two discrete anatomical compartments; the mesenteric domain and the non-mesenteric domain (Figure 2) (5,7). In the mesenteric domain, all digestive organs are directly connected to one, continuous mesentery (5-7). In the non-mesenteric domain, all genitourinary organs are positioned on the musculoskeletal frame of the abdomen (Figure 2) (5,6,8).
The MM is a new foundation on which to base all future scientific and clinical exploration of the abdomen. The word “foundation” is centrally important in science and clinical practice. In order to understand a biological structure, it is essential to know its fundamental components and how these are organised (9). The fundamental contents of a structure, and the organisation of these, are the “foundation” of that structure. The organisation at the foundation level, is the fundamental order. Understanding the foundation of a structure enables one build an accurate model of the entirety of that structure.
The key concepts of foundation and fundamental order apply to abdominal anatomy as much as they apply to any science (5-7). This is explained as follows. As per the MM, the organs of the mesenteric domain are positioned on the mesenteric frame. The organs of the non-mesenteric domain are positioned on the musculoskeletal frame (Figure 2). If digestive organs of the mesenteric domain are conceptually subtracted from that domain then only the mesenteric frame remains (5). If genitourinary organs of the non-mesenteric domain are conceptually removed, the musculoskeletal frame remains. The mesentery and musculoskeletal frame are thus the fundamental components of the abdomen. The organisation of these relative to each other, is the fundamental order of the abdomen. The fundamental components and the order of these, correspond to the anatomical foundation of the abdomen (5).
With an understanding of the anatomical foundation of the abdomen, it is possible to set out the aims of this article. The first aim is to broadly describe the anatomy of abdomen, based off the foundation provided in the MM. The reader will immediately see that abdominal anatomy is far simpler than previously understood to be. The second aim of the article is to show how that anatomy provides a structural basis for all clinical practice related to the abdomen. At completion of the article, students should be in a position to systematically approach any abdominal disease in a mesenteric-based manner. The student should then be able to take their understanding directly back to the clinical setting, and employ it in the clinical process of diagnosing and treating abdominal disease (10,11).
The mesenteric domain
The mesenteric domain includes the mesentery and all abdominal digestive organs (5,6) (Figure 3). The mesentery is currently defined as the organ on which all abdominal digestive organs develop and remain directly connected to (Figure 3) (12,13). This contrasts with the definition used in conventional descriptions of abdominal anatomy, i.e., that the mesentery is a double fold of peritoneum (14,15). The following is a broad explanation of the anatomy of the mesentery. A detailed explanation is provided in the supplementary notes in the manuscript by Byrnes et al. (5).
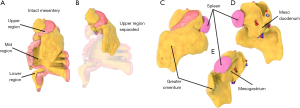
The upper region of the mesentery
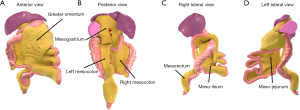
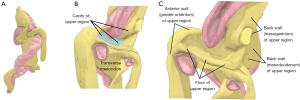
The mesentery is subdivided into three regions (upper, mid and lower) by the mid-region fold (Figure 4) (5). The upper region is sack shaped. The posterior surface of the sack is adherent to the non-mesenteric domain of the upper abdomen. The front wall is made up of the greater omentum and stomach. The posterior wall is the mesogastrium. The floor of the upper region is adherent to the upper surface of the transverse mesocolon (Figure 5). The spleen is directly connected to the left lateral aspect of the upper region sack (Figure 4). The components of the upper region are best visualised by examining sagittal sections of the upper region from both right and left sides (Figure 5).
The mid-region of the mesentery
To understand the shape of this region, one needs to follow the development of it. It is simply not possible to understand the fold-shaped nature of the mid-region of the mesentery otherwise. During development, the mid-region extends forward into the abdominal cavity as a fold with right and left sides (Figure 6). The zone closest the posterior abdominal wall can be called the central zone. The zone furthest from the posterior wall can be called the peripheral zone. At first the right and left sides of the fold remain aligned from central to peripheral zones. With further development a switch occurs at the periphery of the fold. After the switch, the original right side of the periphery is now positioned on the left, and the original left side is now positioned on the right. The sides of the central zone remain unchanged in position. After the switch, a further left to right folding is superimposed on the original right side of the fold. This means that the whole periphery of the mid-region fold bends diagonally from left to right, across the posterior abdominal wall. This organisation is retained throughout life (Figure 6).
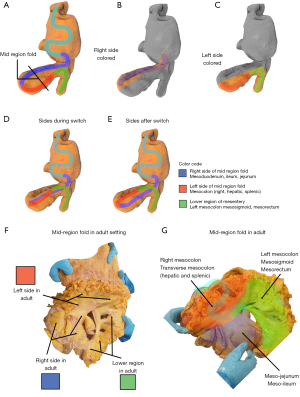
The shape of the mid-region of the mesentery in the adult is explained by the limited number of mesenteric events described in the preceding paragraph. In the adult, the right side of the fold includes the mesoduodenum, mesojejunum and meso-ileum. The left side includes the right and transverse mesocolon (which can be divided into hepatic and splenic regions). Centrally, the mesoduodenal component of the right side (i.e., mesoduodenum) is positioned on the right of the superior mesenteric artery (SMA). Peripherally, the meso-jejunal and meso-ileal components of the right side are to the left of the SMA (Figure 6).
Peripherally, the right mesocolic component of the left side of the mid-region fold is on the right of the SMA. The hepatic component crosses from the right to the left of the SMA and then continues as the splenic component of the transverse mesocolon. The splenic component is the central zone of the original left side. It continues distally as the left mesocolon (Figure 6).
As mentioned above, the original right side comprises the mesoduodenum centrally, the meso-jejunum and meso-ileum peripherally. At the junction between the mesoduodenum and mesojejunum, the right side undergoes a further folding from left to right, towards the right side of the abdomen (Figure 6). As a result, the main bulk of the mid-region fold crosses from left of the midline to the right iliac fossa. This is one of the reasons why the ileocecal junction (and appendix) is normally positioned in the right iliac fossa (Figure 6).
The lower region of the mesentery
This includes the left mesocolon, mesosigmoid and mesorectum (16,17) (Figure 7). The left mesocolon is the continuation of the splenic component of the transverse mesocolon. It continues distally as the mesosigmoid which in turn continues into the pelvis as the mesorectum (18,19). The left colon is at the periphery the left mesocolic region of the mesentery. The sigmoid colon is at the periphery of the mesosigmoid and the rectum is encased by the mesorectum. The upper and mid-level rectum are encased posteriorly and postero-laterally by mesorectum. The lower rectum is encased circumferentially by mesorectum (Figure 7).
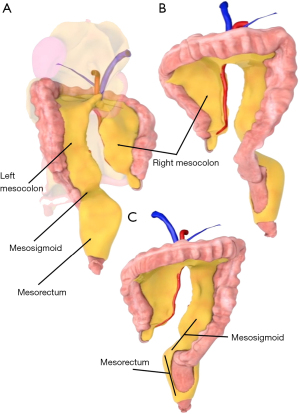
The termination of the mesorectum is the distal anatomical end of the mesentery (Figure 7). The abdominal mesentery commences at the oesophago-gastric junction from which it fans out as the upper region. Hence, the abdominal mesentery has clearly defined start and end points (5).
The MM of the abdomen explains the peritoneum
Anatomists have long grappled with the terms “mesentery” and “peritoneum”. This is reflected in the fact that according to conventional descriptions, the “mesentery” is a double fold of “peritoneum”. If one adheres to that definition then only three regions of the mesenteric continuum described above satisfy the definition and can be termed mesentery (Figure 1). The peritoneal landscape that emerges in that model is thus dauntingly complex. When the peritoneum is interpreted according to the MM, a simpler picture emerges that matches clinical observations (Figures 1,8).
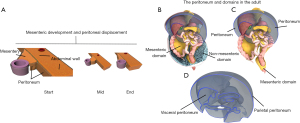
To understand how the MM explains the anatomy of the peritoneum, it is again important to start with the development of the mesentery, the peritoneum and the posterior abdominal wall. Early during development these are all continuous. Soon after, the body of the mesentery separates from the posterior abdominal wall but remains adherent to the latter (Figure 8). As these remain apposed, but separate, an important interface arises between both. Throughout development, the surface lining of the mesentery and abdominal wall remains continuous. This is the peritoneum. The junction between the peritoneal surface lining of the mesentery and that of the abdominal wall is called the reflection. During development, the mesentery adheres across the posterior abdominal wall, lengthening the interface between both, moulding the overlying peritoneum, and displacing the reflection (Figure 8A). At completion of development, many regions of the mesentery, and conjugate organs of it, have both peritonealised and non-peritonealised surfaces.
At completion of development the adult conformation is apparent (Figure 8B-8D). In the adult, regions of the peritoneum are categorised as visceral and parietal. When interpreted in light of the MM, visceral peritoneum corresponds to the surface lining of the free surface (i.e., the non-adherent surface) of the mesenteric domain. The parietal peritoneum is the surface lining of the free surface of the non-mesenteric domain. The reflection is the junction between the visceral and parietal peritoneum (Figure 8).
According to conventional descriptions of abdominal anatomy, the peritoneal landscape is described in terms of sacs, recesses, pouches, cavities and fossae. These lack defined boundaries. Where boundaries are described, they are termed ligaments, mesenteries, reflections, membranes and folds. If these terms are considered in the light of the MM, then the sacs, fossae, cavities, spaces and pouches of conventional descriptions will be seen to arise as the surface landscape of the visceral and parietal peritoneum as explained in the MM (8). The ligaments, folds, reflections and membranes correspond to adaptations of the reflection bridging both regions of peritoneum (7). Thus, the MM explains the organisation of the peritoneum in general, and the peritoneal landscape in particular (Figure 8).
The MM can be used to explain the positional anatomy of all abdominal digestive organs
To understand the organisation of the abdomen, it is necessary to understand the position of each content of it. The position of organs is normally described by referencing surrounding organs or major vessels. This approach fails if the organs used for reference have changed through either surgical or non-surgical (i.e., disease related) means. In conventional approaches, organ position is further qualified using the terms “retroperitoneum”, “extra-peritoneal” and “intraperitoneal” (20,21). None of these terms can be precisely defined using conventional approaches to abdominal anatomy. Hence, the conventional approach to describing organ position is problematic and unstable, and is not rigorous enough to support the level of accuracy and reproducibility required in the clinical setting.
The MM can be applied to explain clearly and reproducibly, the position of all abdominal digestive organs. It is not challenged by the limitations described above. The position of digestive organs can be described in terms of the region of mesentery to which they are directly connected (5) (Figure 9). For example, the duodenum is at the periphery of the mesoduodenal region of the mesentery. The jejunum and ileum are at the periphery of adjoining regions of mesentery (i.e., the meso-jejunum and meso-ileum). The position of the intestine distal to the ileum can also be described in terms of the region of adjoining mesentery (Figure 9). Thus, digestive organ position can be simplified by specifying the region of the mesentery to which the organ is connected.

The MM can be used to explain the positional anatomy of digestive organ vasculature
The same principle applies to defining the position of the vasculature that supports each organ (Figure 10) (5,8). The position of all vessels of the abdominal digestive vasculature can be described in mesenteric terms. This will be illustrated using the coeliac trunk as an example. Once the coeliac trunk enters the mesentery it divides into three major branches. The course of each of these can be described in terms of the region of the mesentery in which it travels (Figure 10). The splenic artery travels in the mesogastrium towards the hilum of the spleen. The hepatic artery travels in the lower pole of the neck of the upper region of the portal pedicle. The hepatic artery enters this zone of the mesenteric continuum to travel with the portal vein to the hilum of the liver.

The course of the veins of the digestive system can be explained in mesenteric terms (Figure 10) (5,8). The superior mesenteric vein travels centrally between the right and left sides of the periphery of the mid-region fold. At the central region of the mesentery it enters the mesoduodenal region of the mesentery behind the pancreas. The inferior mesenteric vein travels proximally in the left mesocolon towards the central region of the mesentery. The splenic region of the transverse mesocolon is the central zone of the mid-region fold on the left. There, the inferior mesenteric vein travels at the medial margin to reach the apex of the fold behind the pancreas. At that level, it is joined by the splenic vein located in the mesogastrium (Figure 10). The newly formed vein then joins the superior mesenteric vein in mesentery posterior to the neck of the pancreas, to form the portal vein. This area of the mesentery is particularly important given the confluence of these vessels. It corresponds to the inferior pole of the neck of the upper region. It also corresponds to the anatomical junction between upper and mid-regions of the mesentery. From here the portal vein enters the portal pedicle (see above) to reach the liver.
As can be seen from the above, the anatomical course of arteries and veins of the abdominal digestive system can be described in terms of the region of the mesentery in which they travel (Figure 10). Digestive system lymphatics and nerves are anatomically associated with arteries (22,23). This observation, taken in combination with the above, means the course of digestive system lymphatics and nerves is also likely to follow the MM (Figure 11).
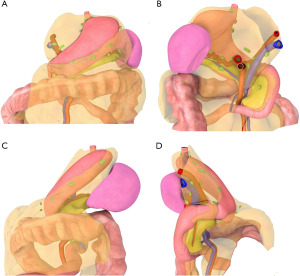
The anatomical relationship between the mesenteric and non-mesenteric domain
The structural relationship between the mesenteric and non-mesenteric domain is centrally important in abdominal surgery, radiology and pathology (Figure 8) (11,24,25). Understanding this relationship enables the reader understand what the abdominal surgeon is doing during organ resection (17). It aids the radiologist in interpreting the radiological appearance of the abdomen (24). It helps the pathologist understand how diseases spread within and effect abdominal contents. It is thus essential to understand the anatomy by which the organisation of the domains is maintained.
As the mesentery and mesenteric organs develop, some regions adhere to the posterior abdominal wall. These events lengthen the interface between mesenteric and non-mesenteric domain, and mould the peritoneum that overlaps the inner surface of both domains (Figures 8,12). The resultant anatomy is then maintained by three mechanisms. The first, the peritoneal reflection, is a direct anatomical and structural connection between the mesenteric and non-mesenteric domain (see above). Connections between domains also occur at the coeliac trunk, superior and inferior mesenteric arteries, and the hepatic veins (Figure 13). The entire arterial inflow to the mesenteric domain normally occurs via just three vessels; the coeliac trunk, superior and inferior mesenteric artery (Figure 13). The entire venous drainage occurs through the hepatic veins into the inferior vena cava. The peritoneal reflection is located at the periphery of the mesenteric domain (Figure 12). The vascular connections are centrally located in the midline (Figure 13). In between central and peripheral connections, the domains are also adherent and a connective tissue layer occupies the interface between both (Figure 12). Regions of the mesenteric domain that are adherent to the non-mesenteric domain, include (from top to bottom) the mesogastrium, mesoduodenum, right and left mesocolon, medial mesosigmoid and mesorectum. Some areas of the colon, liver and spleen are also adherent to the non-mesenteric domain.

How the MM applies in clinical practice
As mentioned above, the second aim of this article is to demonstrate, how the MM applies to clinical practice. Abdominal disease follows the MM. The distribution of abdominal disease, is explained by the MM. For example, tumors emerge in a domain and then spread in that domain. This is why rectal tumors metastasise to the liver, but not to the kidney, and vice versa. Intraabdominal collections (common consequences of inflammation or perforation) follow the peritoneal landscape. The anatomy of the peritoneal landscape is explained by the MM (see above). Fluid collections in pancreatitis challenge radiologists as to where these are located. This arises out of adherence to the conventional model of abdominal anatomy which holds that the left and right regions of the mesocolon are normally absent. Fluid collections in pancreatitis are explained by their accumulation in the interface between the mesenteric and non-mesenteric domain. The secondary effects of abdominal inflammation (a feature of almost every abdominal disease), are explained by the MM.
Given the distribution of all abdominal disease follows the MM, then the radiological interpretation of abdominal disease, and the surgical intervention for abdominal disease, are optimal when based off the MM. The next sections explain how the MM applies in the diagnosis and treatment of disease. It follows that the interpretation of abdominal disease, based on the tenets of the MM, best equips the student and clinician in the diagnosis and management of clinical abnormalities.
Application of the MM to abdominal surgery
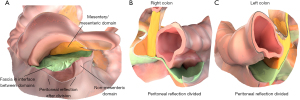
The MM is directly relevant to all techniques in resectional colorectal surgery. When excising a rectal cancer, the surgeon removes the mesorectum and adjoining rectum (26,27). To achieve this, the mesorectum is detached from the surrounding non-mesenteric domain. When excising a colon cancer, the surgeon removes the mesocolon and adjoining colon (28). To do this, the surgeon accesses the interface between the overlying mesocolon and underlying abdominal wall. The surgeon will then detach the mesocolon from the fascia and hence abdominal wall (Figure 12).
The interface between the mesentery and abdominal wall is not apparent when the surgeon first enters the abdominal cavity. The interface must be exposed by dividing the peritoneal reflection (11,29-31) (Figure 12). After division of the reflection, the surgeon lifts the mesenteric domain away from the posterior abdominal wall. This action places the interface between domains under stretch. This has the effect of exaggerating it, making it is visible to the surgeon. The mesocolon (i.e., a region of the mesenteric domain) can then be peeled away from the underlying posterior abdominal wall (i.e., a region of the non-mesenteric domain) (Figure 12) in a reverse of the embryological event that led to the anatomical arrangement in the first instance.
Division of the reflection and separation of the mesenteric from non-mesenteric domain are core activities in general and intestinal surgery. They are grouped under the term “mesenteric-based surgery”. This approach is now increasingly being adopted in surgery for gastric, pancreatic and oesophageal malignancy. It is essential in multivisceral transplantation, in which the entire mesenteric domain is detached from the posterior abdominal wall. Following detachment the mesenteric domain is then disconnected and transplantation is completed by restoring connections in the recipient.
When the aorta and inferior vena cava must be quickly accessed, such as in trauma, vascular surgeons conduct a “medial or lateral visceral rotation” to rapidly access and secure major vessels. During this procedure, the reflection is divided and the mesenteric domain is then peeled off the posterior abdominal wall, from lateral to medial or vice versa. The inferior vena cava and abdominal aorta are exposed and the surgeon can directly repair these as they are no longer impeded by organs of the mesenteric domain.
Urological surgeons separate the mesenteric domain from the posterior abdominal wall to access the kidneys and ureters, i.e., contents of the non-mesenteric domain. The urological surgeon divides the reflection, separates the colon and adjoining mesocolon from the underlying posterior abdominal wall and thus accesses a zone previously termed the “retroperitoneum”.
How surgeons excise a digestive organ—in two easy steps
For decades students have observed surgeons conducting abdominal operations and struggled to reconcile what they were seeing being done, with conventional anatomical descriptions. The operative landscape bore little resemblance to the anatomical landscape the student was expecting. The main reason for this is that over the past century, surgical practice departed from conventional anatomical roadmaps to become almost entirely mesenteric-based. The recent articulation of the MM now enables us reconcile abdominal anatomy with surgical anatomy. This means the student can now study abdominal anatomy and prepare themselves appropriately to interpret the landscape they encounter intraoperatively.
With this in mind, the following is a MM based anatomical description of the techniques used by surgeons to excise abdominal digestive organs. The following applies even in the most challenging of contexts (i.e., in the disease or re-operative settings or in the setting of congenital abnormalities). Excision of any organ of the mesenteric domain can always be simplified (reduced) to two steps:
- Separation of that region of the mesenteric domain that is to be resected, from the non-mesenteric domain to which it is attached.
- Disconnection of the region of the mesenteric domain to be resected, from the rest of the mesenteric domain.
Splenectomy (removal of the spleen) is used as an example to illustrate the steps involved:
- Separation of the spleen (i.e., a region of the mesenteric domain) from the non-mesenteric domain.
The splenic region of the mesenteric domain is connected to the non-mesenteric domain by the peritoneal reflection at the border of the spleen. Once this is incised and divided, the region of the mesentery to which the spleen is connected can be detached from the posterior abdominal wall. This region of the mesentery is the mesogastrium (Figures 3,4). Once the spleen and mesentery have been mobilised, the surgeon enters the cavity of the upper region by dividing through the greater omentum. The anterior surface of the mesogastrium comes into view. The splenic recess of the upper region sack is apparent to the left, and can be divided through. By this point, that zone of the upper region sack to which the spleen is connected, has been mobilised from the surrounding non-mesenteric domain. The term “mobilised” means a structure is mobile enough to allow unimpeded dissection through it. That dissection is required to surgically expose, clamp, divide, and ligate vessels contained within the structure. - Disconnection of the spleen (i.e., region of the mesenteric domain to be resected), from the rest of the mesenteric domain.
In the case of solid organs, this simply involves division of the mesentery to which the organ is directly connected. Following division, the organ can be removed together with adjoining mesentery. For regions of the intestine, the mesentery must be divided, and the intestine must be disconnected from adjoining intestine.
During division of the mesentery to which an organ is a connected, it is important to address blood vessels in that region of mesentery. As mentioned above, the entire vasculature (arterial and venous) of abdominal digestive organs is located in the mesentery. Knowing, broadly speaking, which vessels to expect, the surgeon can carefully explore the mesentery to expose, clamp, divide and ligate these vessels. For example, the splenic vein and artery are located in the mesogastrium, posterior to the pancreas. These travel towards the hilum of the spleen in the mesogastrium. At the hilum they can be exposed and divided. The spleen is also supplied by the short gastric vessels in the greater omentum. By haemostatically dividing the greater omentum, these are also divided.
Application of the MM to abdominal radiology
At present, most radiologists adopt the conventional, peritoneal model when exploring and explaining the radiological appearance of the abdomen, i.e., the appearance of the abdomen on computed tomography (CT) or magnetic resonance imaging (MRI). Increasingly, abdominal radiologists are adapting the MM, to interpret and explain the radiological appearance of the abdomen (24,25,32).
Using the MM, the abdominal radiologist can now build a composite and comprehensive digital simulation model of the abdomen (Figure 14). This was not possible heretofore, as several regions of the mesentery were considered misnomers (i.e., several pieces of the jigsaw were omitted). For example, the mesorectum, mesocolon, mesogastrium and mesoduodenum are all apparent on radiological imaging of the abdomen. None of these is acknowledged in Terminologica Anatomica 2, which is the list of terms anatomists use to describe the components of the body (33). Given these radiological regions lack a corresponding terminology, they lack an anatomical correlate by conventional descriptions. These challenges are immediately overcome when radiological interpretations of the abdomen are based off the MM and the concept of a mesenteric continuum.
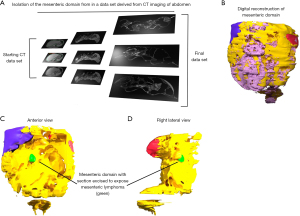
Ultrasound can be combined with endoscopy to identify the mesentery during endoscopy. This is called endoscopic mesenterography and increasingly is being investigated in relation to the lower region of the intestine (6). The hypothesis is that if the mesentery can be identified (by ultrasound) at endoscopy, then it is possible to sample it. This would involve a process similar to transrectal biopsy of the prostate. If it is possible to sample it, then it would also be possible to target it therapeutically, in a manner similar to that involved in prostatic brachytherapy.
Application of the MM to abdominal pathology
In general, pathologists adhere to the peritoneal or conventional approach to abdominal anatomy. This presents challenges as they are required to harvest nodes from regions of mesentery that, according to the conventional model, are not normally present in the adult human (i.e., the mesogastrium, mesoduodenum, mesocolon and mesorectum). The MM now presents the pathologist with a far simpler and clinically applicable reference when harvesting lymph nodes and mapping disease spread (Figure 11).
During post-mortems, pathologists generally tend to remove abdominal digestive organs as an intact unit. This approach is based off one originally developed by Rokitansky and is mesenteric based. During the Rokitanski approach the pathologist divides the peritoneal reflection, separates the mesenteric from non-mesenteric domain, and then disconnects these. The underlying mesenteric basis to this approach cannot be appreciated by adhering to the conventional, peritoneal based, model of abdominal anatomy.
Knowledge of the shape of the mesentery, and its connections with abdominal digestive organs, helps us better understand how diseases spread locally, regionally and remotely to distant organs. As mentioned above, tumours generally remain domain specific and spread in a domain specific manner (6,26). It is unusual (though not rare) for a rectal or colonic tumour to directly spread to involve the adjacent non-mesenteric domain. Such tumours are categorised as “T4” in the American Joint Committee on Cancer (AJCC) tumour staging system. “T4” spread generally signifies perforation of a tumour of the mesenteric domain and unsurprisingly carries a poor prognosis.
It is not known why tumour spread is largely domain-based. The domain specific nature of tumor spread may be explained by the relationship of the mesentery to digestive organs. The liver and rectum are directly linked by the mesentery. Metastases from colorectal cancer are frequently observed in nodes in the left or right mesocolon and also at the level of the portal pedicle (see above). For example, this explains the finding of metastatic lymph nodes at the portal pedicle (i.e., adjacent the liver) in patients with rectal cancer. The mesentery thus provides a direct route which metastatic cells from an intestinal malignancy, can employ to transit along (i.e., spread) and reach the liver. The tendency for malignant tumor to exploit the mesentery, and spread in a domain specific manner, is important. It means that surgeons can achieve good long term survival rates if they remove (I) the organ containing a tumour, and (II) the mesentery adjoining that organ.
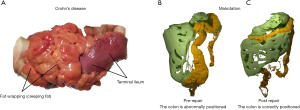
Many diseases that previously lacked a comprehensive explanation can now explained using a mesenteric-based approach. Resection of the mesentery in Crohn’s disease is increasingly being adopted to reduce recurrence and the requirement for reoperation in this patient cohort (Figure 15). Up to recently, malrotation was regarded as an intestinal disease. This condition is one of the most dreaded surgical conditions in the neonatal period. The cause of malrotation can be understood if the development of the mesentery is taken into account. During development the sides of the mid-region undergo a switch in position relative to the SMA. If that switch does not occur, the sides remain aligned from central to peripheral zones (Figure 15). When aligned, they fail to adhere to the non-mesenteric domain and thus are prone to twisting around the SMA. This results in twisting of the intestine and adjoining mesentery and can be fatal unless surgically resolved.
Loop formation during colonoscopy is one of the commonest causes of pain and failure of colonoscopy. This event is understood by examining the changes in shape which both the intestine and mesentery undergo. As the intestine lengthens between two fixed points it adopts a curved shape and the adjoining mesentery buckles. As the intestine lengthens further, the intestinal curve progresses to a coil (i.e., a loop). The adjoining mesentery must follow and thus forms a spiral. If attempts are made at further advancement of the scope, both intestine and mesentery stretch and the patient experiences pain.
The anatomical changes that occur during loop formation, also occur during volvulus. Conventional descriptions emphasise intestinal twisting and largely ignore the effects on the adjoining mesentery.
Volvulus of the intestine was classically attributed to “abnormal persistence of a mesentery and increased mobility of the intestine”. As it is now accepted that the mesentery persists at all levels, it is necessary to reappraise our understanding of the cause of volvulus. Volvulus tends to occur in two settings. Ileocecal volvulus occurs mainly in younger patients whilst sigmoid volvulus normally occurs in older patients. In ileocecal volvulus, the ileocecal region of the mesenteric domain is always mobile. Normally during development, the right and left mesocolon progressively adhere to the posterior abdomen wall. As they do so, they displace the peritoneum to the lateral extremities of the abdomen. If adhesion does not occur (i.e., mal-adhesion) then the mesentery and adjoining intestine remain mobile. Thus, ileocaecal volvulus is more likely explained by failure of adhesion of the mid-region of the mesentery to the posterior abdominal wall.
In the setting of elderly patients, volvulus arises from hypermobility of the mesosigmoid. Normally, the medial part of the mesosigmoid is adherent to the posterior abdominal wall. This usually prevents the mobile part of the mesosigmoid from undergoing torsion or volvulus. As one ages, the mesosigmoid and intestine continue to lengthen. Lengthening is conspicuously pronounced in patients with neurological abnormalities such as Parkinson’s disease. At a certain tipping point, the mobile region of the mesosigmoid overcomes the anchorage effect of the adherent medial part, and the mesosigmoid and sigmoid undergo torsion.
Summary
In this article we briefly explained the MM of abdominal anatomy and how disease follows this model. The model substitutes the conventional peritoneal-based model that depicted the abdomen as a complex organisation. At this point it is hoped the student understands the anatomy of the abdomen in broad terms. In addition, it is also hoped the student can better understand abdominal disease. That being the case, then the student is automatically better equipped to detect and treat abdominal disease.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://map.amegroups.com/article/view/10.21037/map-22-2/coif). JCC serves as the Editor-in-Chief of Mesentery and Peritoneum. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coffey JC, O'Leary DP. The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol 2016;1:238-47. [Crossref] [PubMed]
- Byrnes KG, McDermott K, Coffey JC. Development of mesenteric tissues. Semin Cell Dev Biol 2019;92:55-62. [Crossref] [PubMed]
- Dodds WJ. Retroperitoneal compartmental anatomy. AJR Am J Roentgenol 1987;148:829. [Crossref] [PubMed]
- Dodds WJ, Darweesh RM, Lawson TL, et al. The retroperitoneal spaces revisited. AJR Am J Roentgenol 1986;147:1155-61. [Crossref] [PubMed]
- Byrnes KG, Walsh D, Walsh LG, et al. The development and structure of the mesentery. Commun Biol 2021;4:982. [Crossref] [PubMed]
- Coffey JC, Byrnes KG, Walsh DJ, Cunningham RM. Update on the mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol 2022;7:96-106. [Crossref] [PubMed]
- Coffey JC, Byrnes KG, Walsh D, et al. The mesentery and the mesenteric model of abdominal compartmentalisation. Gray's Anatomy. Commentary 2020;8:1.
- Coffey JC, Walsh D, Byrnes KG, et al. Mesentery - a 'New' organ. Emerg Top Life Sci 2020;4:191-206. [Crossref] [PubMed]
- Coffey JC. Surgical anatomy and anatomic surgery - Clinical and scientific mutualism. Surgeon 2013;11:177-82. [Crossref] [PubMed]
- Sehgal R, Coffey JC. Historical development of mesenteric anatomy provides a universally applicable anatomic paradigm for complete/total mesocolic excision. Gastroenterol Rep (Oxf) 2014;2:245-50. [Crossref] [PubMed]
- Coffey JC, Dillon M, Sehgal R, et al. Mesenteric-Based Surgery Exploits Gastrointestinal, Peritoneal, Mesenteric and Fascial Continuity from Duodenojejunal Flexure to the Anorectal Junction--A Review. Dig Surg 2015;32:291-300. [Crossref] [PubMed]
- Byrnes KG, McDermott KW, Coffey JC. Mesenteric organogenesis. Semin Cell Dev Biol 2019;92:1-3. [Crossref] [PubMed]
- Byrnes KG, Walsh D, Dockery P, et al. Anatomy of the mesentery: Current understanding and mechanisms of attachment. Semin Cell Dev Biol 2019;92:12-7. [Crossref] [PubMed]
- Carmichael JC, Mills S. Anatomy and Embryology of the Colon, Rectum, and Anus. In: Steele SR. editor. The ASCRS textbook of Colon and Rectal Surgery: Springer International Publishing; 2016:3-26.
- Gray H. Anatomy, descriptive and Surgical. 1 ed. New York; 1858.
- Coffey JC, Dockery P, Moran BJ, et al. Mesenteric and peritoneal anatomy. In: Coffey JC. editor. Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science. 1. Boca Raton, Florida, USA: CRC Press, Taylor & Francis Group; 2017:11-40.
- Coffey JC, Dockery P. Peritoneum, mesentery and peritoneal cavity. In: Brennan PA, Standring S, Wiseman SM. editors. Gray's Surgical Anatomy. 1. 1 ed. Poland: Elsevier; 2020:418-26.
- D'Souza N, Lord A, Shaw A, et al. The sigmoid take-off: An anatomical imaging definition of the rectum validated on specimen analysis. Eur J Surg Oncol 2020;46:1668-72. [Crossref] [PubMed]
- D'Souza N, Lord AC, Shaw A, et al. Ex vivo specimen MRI and pathology confirm a rectosigmoid mesenteric waist at the junction of the mesorectum and mesocolon. Colorectal Dis 2020;22:212-8. [Crossref] [PubMed]
- Oliphant M, Berne AS. Computed tomography of the subperitoneal space: demonstration of direct spread of intraabdominal disease. J Comput Assist Tomogr 1982;6:1127-37. [Crossref] [PubMed]
- Meyers MA, Oliphant M, Berne AS, et al. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology 1987;163:593-604. [Crossref] [PubMed]
- Hogan J, Chang KH, Duff G, et al. Lymphovascular invasion: a comprehensive appraisal in colon and rectal adenocarcinoma. Dis Colon Rectum 2015;58:547-55. [Crossref] [PubMed]
- Culligan K, Sehgal R, Mulligan D, et al. A detailed appraisal of mesocolic lymphangiology--an immunohistochemical and stereological analysis. J Anat 2014;225:463-72. [Crossref] [PubMed]
- Dalla Pria HRF, Torres US, Velloni F, et al. The Mesenteric Organ: New Anatomical Concepts and an Imaging-based Review on Its Diseases. Semin Ultrasound CT MR 2019;40:515-32. [Crossref] [PubMed]
- Coffey JC, Culligan K, Walsh LG, et al. An appraisal of the computed axial tomographic appearance of the human mesentery based on mesenteric contiguity from the duodenojejunal flexure to the mesorectal level. Eur Radiol 2016;26:714-21. [Crossref] [PubMed]
- Bunni J, Coffey JC, Kalady MF. Resectional surgery for malignant disease of abdominal digestive organs is not surgery of the organ itself, but also that of the mesenteric organ. Tech Coloproctol 2020;24:757-60. [Crossref] [PubMed]
- Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med 1988;81:503-8. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 2009;11:354-64; discussion 364-5. [Crossref] [PubMed]
- Coffey JC, Dillon MF, O'Driscoll JS, et al. Transanal total mesocolic excision (taTME) as part of ileoanal pouch formation in ulcerative colitis--first report of a case. Int J Colorectal Dis 2016;31:735-6. [Crossref] [PubMed]
- Culligan K, Coffey JC, Kiran RP, et al. The mesocolon: a prospective observational study. Colorectal Dis 2012;14:421-8; discussion 428-30. [Crossref] [PubMed]
- Culligan K, Walsh S, Dunne C, et al. The mesocolon: a histological and electron microscopic characterization of the mesenteric attachment of the colon prior to and after surgical mobilization. Ann Surg 2014;260:1048-56. [Crossref] [PubMed]
- Coffey JC, O'leary DP. Defining the mesentery as an organ and what this means for understanding its roles in digestive disorders. Expert Rev Gastroenterol Hepatol 2017;11:703-5. [Crossref] [PubMed]
- FIPAT. Terminologia Anatomica 2. In: Terminology. FIPFA, editor. Second ed. Available online: FIPAT.library.dal.ca; 2019.
Cite this article as: Lim EMY, Baban CK, Peirce CB, Walsh D, Coffey JC. Abdominal anatomy—the foundation of clinical practice. Mesentery Peritoneum 2022;6:1.


